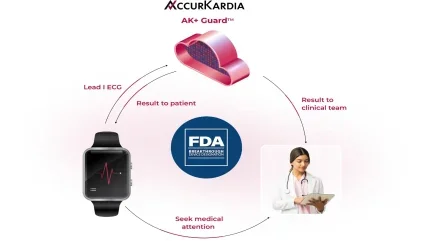
AccurKardia, an innovator in ECG-based diagnostics technology, has announced that it has received Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its ECG-based, AI-powered AK+ Guard hyperkalemia detection software. The investigational technology uses Lead I ECG data to alert patients and clinicians of moderate to severe episodes of hyperkalemia (excess potassium in the blood) that can lead to sudden cardiac arrest.
AK+ Guard is designed to work with a wide range of FDA-cleared consumer and clinical wearables that currently capture Lead I ECG data (e.g., smartwatches), enabling hyperkalemia monitoring outside of the clinic and earlier intervention for high-risk populations. These include those with end stage renal disease, chronic kidney disease (CKD), and other risk factors.
AK+ Guard was also recently accepted as one of only 62 devices in the FDA Total Product Life Cycle Advisory Program (TAP). The TAP program provides earlier and more frequent engagement with the FDA, helping accelerate the regulatory process, improve the quality and timeliness of device evaluations, and ultimately speed commercialization.
“AccuKardia’s AK+ Guard has the potential to be a game changer in the early detection of moderate to severe episodes of hyperkalemia, a life-threatening yet often asymptomatic condition that currently can only be diagnosed via blood-draw to directly measure potassium levels,” said Wei Ling Lau, MD, Interim Chief of the Division of Nephrology, Hypertension & Kidney Transplantation at the University of California, Irvine. “The company’s novel solution could offer a convenient way to extend hyperkalemia monitoring to the home for vulnerable patients.”
There are 37 million people in the U.S. that suffer from CKD, and in 2024, 518,970 patients were on dialysis. Among CKD patients, hyperkalemia is associated with a 16.6 percent increase in all-cause mortality. The risk is even more pronounced in dialysis patients: the rate of all-cause mortality increases by 33 percent after a moderate to severe hyperkalemia episode, and as many as 17 percent of dialysis patients experience these episodes annually. Moreover, for patients with CKD, the one-year healthcare cost associated with hyperkalemia is $25,156, and the average hospital stay cost for a patient with hyperkalemia, published most recently in 2014, was $29,181 with an average hospital length of stay of 3.3 days.
“The two FDA actions supporting AK+ Guard mark another major milestone in AccurKardia’s journey towards achieving our mission to improve patient outcomes and save lives by transforming ECG into a broad biomarker,” said Juan C. Jimenez, co-founder and CEO of AccurKardia. “We believe the current standard of care for hyperkalemia detection and monitoring is underserving patients, and we aim to deliver a speedier and more accessible pathway to detection and risk management that will make a meaningful impact on patient care.”
The AK+ Guard news follows AccurKardia’s recently received Breakthrough Device Designation for its Aortic Valve Stenosis (AVS) screening software, AK-AVS, which is designed to leverage the ubiquity of the electrocardiogram (ECG) to identify potential cases of aortic valve stenosis within millions of ECGs already present in healthcare system electronic health records. AK-AVS software aims to help identify and prioritize which patients should receive echocardiograms for definitive diagnosis.






