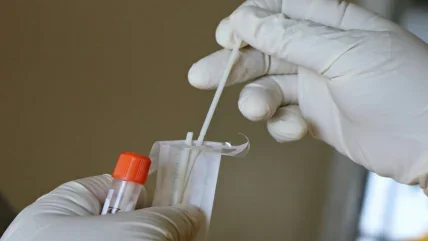
In April 2021, the UK government urged citizens to swab their nasal passages twice a week. Even people without symptoms could test themselves for the presence of SARS-CoV-2, the virus that causes Covid-19, with freely available diagnostic kits. Swabbers would get a result within 30 minutes to help them better understand their risk of transmitting the pathogen to others. Although the test is not as accurate as the gold-standard polymerase-chain-reaction (PCR) assay for Covid- 19, it is much faster and more convenient. There’s no need to send the sample to a laboratory; anyone can do this at home.
The kit is an example of a lateral flow test, where a biological sample, diluted in a chemical buffer, wicks through a paper strip by capillary action. In theory, if coronavirus antigens are present in the patient’s sample, a positive band appears on the device’s readout area. Lateral flow tests are a type of microfluidic technology, where low volumes of fluids (such as blood, saliva and urine) are processed and analysed. But these cheap paper tests aren’t suitable for detecting every health condition. Prior to Covid-19, the most common application of lateral flow technology was in home pregnancy tests, but unlike the chorionic gonadotropin (hCG) hormone detected in the urine of pregnant women, the viral load present in a Covid-19 swab can vary significantly. Without an amplification method, such tests are always going to be less sensitive to lower viral loads than RT-PCR. The lack of amplification means that paper lateral flow tests typically have low sensitivity – the ability of the test to correctly identify those patients with the disease – so, a large amount of antigen is required to trigger a positive band. Another challenge is lateral flow tests typically only detect a single biomarker, so they can only be used to rule out one disease at a time. More sophisticated microfluidic tests typically use miniscule channels encased in glass or silicon.
For decades, point-of-care diagnostics have been heralded as the answer to global healthcare challenges. LOC technology could perhaps have the most impact in detecting infectious diseases in developing countries, where healthcare facilities and diagnostic laboratories are in short supply. However, most low-income countries are still yet to see meaningful adoption of LOC tests despite the years of research investment.
Making microfluidics
One reason for the lack of uptake is that the technology required to manufacture sophisticated microfluidic tests is very expensive, points out Robert Hughes from the department of mechanical engineering at the University of Bristol, who studies the mechanics behind point-of-care diagnosis. “The researchers in the developing world often don’t have the agency and the tools to make use of these technologies,” he says.
LOC tests contain micro-scale channels that are often around the width of a human hair. Fluid is controlled, manipulated, and analysed within these channels. But manufacturing the tiny passageways is costly. The most common method involves a process called photolithography, which uses a beam of photons to etch channels into silicon wafers. This technique produces a master mould of the microfluidic system. Next, a biocompatible polymer such as polydimethylsiloxane (PDMS) is poured over the mould, peeled free and then bound to glass. Photolithography can even produce high-resolution channels at the nanometre scale. But the method is time-consuming – and the cost is considerable. This makes the widespread research and development of LOC technology near impossible for all but the wealthiest of research institutes, says Hughes.
“The researchers in the developing world often don’t have the agency and the tools to make use of these technologies.”
Robert Hughes
In fact, photolithography is so expensive that his research grant wouldn’t stretch to it. Instead, he set his sights on 3D printing. “I had a small amount of money, but I wasn’t willing to spend it on something I couldn’t then adapt,” he says. His ultimate aim was to produce microfluidic devices that could be cheaply manufactured and deployed from anywhere, from schools to research facilities in developing countries. He needed to devise a way of making LOC devices as cheaply as possible.
The Bristol team is not the first research group to explore 3D printing for microfluidic channels. Other scientists have trialled additive manufacturing for LOC chip devices and come up against a range of limitations. In 2016, a team from the University of Seattle found that 3D-printed parts could not be arbitrarily joined at the channel intersections. They also discovered that 3D printing resulted in weak seals between the device’s layers. What’s more, the size of the extruded material was often larger than channel diameters typically required in microfluidic point-of- care tests.
“Another problem with 3D printing is that you get lots of ridges as you lay down each filament,” says Hughes. “So, it ends up being a very rough surface, which means that when you come to add the PDMS to make the chips themselves, it’s very hard to stick it to the glass.” The roughness can also lead to contamination and fouling of the channels, meaning they wouldn’t be able to be reused.
Another dimension
Hughes, along with researchers Harry Felton and Andrea Diaz Gaxiola, was determined to find a 3D-printing technique that was fit for purpose. Together they developed a more accessible way to manufacture microfluidic chips using a desktop material extrusion (MEX) 3D printer. They shunned expensive equipment and instead employed an Ultimaker 3 Extended machine (a standard commercially available unit) with a 0.4mm nozzle. They also set about developing open-source software that would allow any user to replicate the process. Their findings are detailed in the journal PLOS ONE.
After much trial and error, the team found a process they were satisfied with. First, they 3D print interconnecting microchannel scaffolds directly on to a build plate (the flat surface that 3D-printed objects will stick to during printing). The channels are then mounted on 1mm-thick glass microscope slides before being heated for around a minute to bind them to the glass. This produces a microfluidic chip master mould. Next, the slides are rapidly cooled on a metal plate before being removed from the mould. The researchers can then use and reuse the master to produce devices made from PDMS. Once the scaffolds emerge from the printer, the master-mould fabrication process takes a matter of minutes, Hughes reveals.
The team managed to print channels 100μm in diameter, which they say is a marked improvement over the standard 3D printing channel resolution. They anticipate the resolutions improving as the field of MEX 3D printing continues to evolve.
Teaching tool
“This technique is so simple, quick and cheap that devices can be fabricated using only everyday domestic or educational appliances and at a negligible cost,” said Felton in the paper’s accompanying press release. “This means researchers and clinicians could use our technique and resources to help fabricate rapid medical diagnostic tools, quickly and cheaply, with minimal additional expertise or resources required.” Clinicians in resource-poor countries would be able to apply the channels directly to any cleaned glass surface, such as a mobile phone screen or car windshield for resourceful point-of-care testing, he added.
The Bristol team say whole libraries of interconnecting channel moulds can be manufactured so cheaply that they can even be used as an educational tool in schools. Hobbyists could make LOC devices using only household equipment with no need for hazardous chemicals. And using the Bristol-developed plug-in, a user can go from a microfluidic channel design to a completed 3D channel without needing CAD software expertise.
The Bristol team now hope to identify potential collaborators in both research and education to help demonstrate the impact the manufacturing technology can have for both medical settings and outreach activities. An obvious next step is to manufacture a specific point-of-care medical test using the team’s 3D-printing technique and test its sensitivity and specificity. Hughes’ team is working with chemists at the university to help make this pursuit a reality.
“Researchers and clinicians could use our technique and resources to help fabricate rapid medical diagnostic tools, quickly and cheaply, with minimal additional expertise or resources required.”
Harry Felton, University of Bristol
“My hope is that we can inspire a whole generation of people about microfluidics,” says Hughes. “I think it’s a fascinating area and I certainly think it’s going to underpin a lot of medical diagnostics in the future.”
The Bristol team’s microfluidic mould-manufacturing process
Robert Hughes’ team from the University of Bristol has developed a low-cost and open-source process for manufacturing microfluidic devices using 3D-printed interconnecting microchannel scaffolds. The basic process is as follows:
- Use open-source software to design microchannel module.
- Print the microchannel scaffolds.
- Assemble the interconnecting channels on glass substrate.
- Bond channels to substrate using heat.
- Rapidly cool on metal plate.
- Peel mould from substrate slide.
A fidget spinner for UTIs
A multidisciplinary and international team of Korean and Indian researchers developed a fidget spinner-based device to detect UTIs from urine samples. The rectangular device, which takes as little as 1ml of urine, was designed to be made cheaply for use in resource-limited settings. One or two nudges causes the device spins for a long time, the motion of which pushes any bacteria on to a membrane. It is then dyed, with a colour change visible in less than one hour that indicates the amount of bacterial load.
The spinner was field tested on 39 patients in Tiruchirappalli, India, all of which would have been given antibiotics based on their symptoms. The researchers found that 59% of the patients were found to be over or under-treated with antibiotics.
Another test gave an initial indication of antibiotic resistance by testing the spun samples treated with different drugs and comparing them to untreated samples. The team was able to quickly decide on which antibiotic might work best to treat the UTI. While this does not compare to lab-based tests, the researchers noted in their paper published in the journal Nature Biomedical Engineering that it is still a useful tool for resource-limited areas that do not typically test for resistance.
The further development of centrifuge-based microfluidics may enable the acceleration of each of the steps of bacterial infection diagnostics, eliminating the need for multiple devices and enabling on-site diagnostics, significantly reducing diagnostic time and overall costs.






