Anaconda Biomed, a developer of neurothrombectomy devices, has announced the first US patient enrolment in its ATHENA clinical trial for the ANA Funnel Catheter.
The first treatment with the device was conducted by Dr. Shahram Majidi at Mount Sinai in New York.
The ATHENA trial is a global, randomised study involving 327 patients to assess the safety and effectiveness of the ANA Funnel Catheter in treating large vessel occlusion acute ischemic stroke.
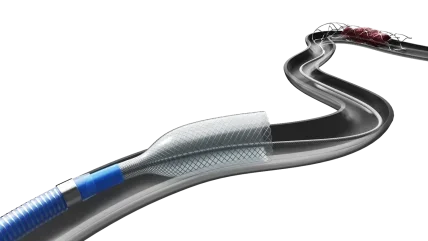
Designed for use during stent retriever-based thrombectomy procedures, the ANA Funnel Catheter aims to facilitate clot removal by restricting blood flow during retrieval. This design enables simultaneous aspiration while reducing the risk of clot fragmentation, said Anaconda Biomed.
Majidi said: “We have long recognised the impact of fast and complete reperfusion in stroke patients, and we are delighted to have initiated this important clinical trial for examining the role that flow restriction with funnel catheter can play in enhancing endovascular thrombectomy and potentially improving outcomes for stroke patients.”
According to Anaconda Biomed, the device features the largest capture diameter currently available in neuroendovascular thrombectomy, which has shown favourable results in prior feasibility studies, demonstrating high rates of reperfusion and success on the first pass. This data supported the company’s investigational device exemption (IDE) submission.
In September 2024, the US Food and Drug Administration (FDA) granted IDE approval for the ATHENA trial to evaluate the ANA Funnel Catheter.
Anaconda Biomed plans to complete patient enrolment by the first half of 2026. The ANA5 funnel catheter is engineered to aid neurovascular procedures by facilitating the delivery of intravascular devices like stent retrievers and microcatheters.
It includes a radiopaque, self-expanding funnel with a continuous sealing coating designed to enable temporary local flow restriction when deployed.
Globally, stroke remains one of the top causes of death and long-term disability, with ischemic strokes accounting for 87% of cases. As populations age, the incidence of stroke is projected to increase significantly, with a 34% rise anticipated by 2035.
Anaconda Biomed’s ongoing clinical trial seeks to address this growing medical challenge by evaluating a device that could potentially improve procedural outcomes in acute ischemic stroke treatment.
Anaconda Biomed CEO Trent Reutiman said: “The ATHENA clinical trial marks the next important step in advancing our funnel catheter technology for ischemic stroke and builds on the promising preliminary efficacy results observed during our earlier ANAIS Study. We are pleased to now be enrolling this vital study in the US while also achieving a fast start internationally.”






