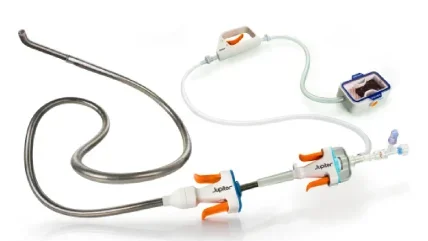
US-based medical technology startup Jupiter Endovascular has completed the treatment of the first US patient using its Vertex Pulmonary Embolectomy System with Endoportal Control platform technology.
The first patient was treated at Staten Island University Hospital, Northwell Health, by Mitchell Weinberg and Vincent Gallo, as part of the SPIRARE II clinical trial.
Jupiter designed the Vertex system to treat Acute Pulmonary Embolism (PE) in an advanced endovascular procedure offering an unprecedented level of control and precision.
The Endoportal Control platform technology integrated into the Vertex system is designed to bring greater ease and stability to a variety of catheter interventions.
The technology allows interventionalists to treat anatomical sites that they cannot safely or easily reach with a conventional endovascular approach.
Weinberg said: “The unique manoeuvrability of the large-bore Vertex endoportal device enabled us to easily navigate across multiple bends through the right heart and into the pulmonary arteries.
“Once in the pulmonary arteries, the technology allowed us to stabilise the endoportal device, creating a secure base for us to safely advance the aspiration catheter deep within the pulmonary vasculature and capture hard-to-reach thrombi.”
Gallo said: “Navigating the pulmonary arteries can be challenging and often requires a complex trial-and-error approach involving multiple guidewires and ancillary devices in order to safely reach the target vessels.
“Using Endoportal Control with the Vertex system, we eliminated many of these extra steps and device exchanges, resulting in a much simpler procedure that allowed us to focus less on gaining vessel access and more on treating the patient.”
SPIRARE II is a single-arm, multicentre clinical trial that enrols up to 145 patients with acute, intermediate-risk PE, treated with the Vertex System, at up to 25 US sites.
The endpoints of the clinical trial include the procedural and clinical benefits of PE treatment with Endoportal Control using the Vertex system.
The benefits are measured across safety, right heart function, and clinical improvement from the time of the procedure to 30 days post-procedure.
Jupiter Endovascular CEO Carl St. Bernard said: “Navigation challenges during endovascular procedures are often underappreciated and have led to under-adoption of life-saving procedures, such as pulmonary embolectomy.
“We have purpose-built our Endoportal Control technology to solve these issues and make important endovascular procedures accessible to more clinicians and their patients who can benefit from them.
“This first case in the U.S. could not have gone better and appears to validate the safety and performance we are seeing in our currently enrolling European SPIRARE I study.”
Last month, Jupiter announced the successful treatment of its first two patients using its Vertex Pulmonary Embolectomy System, as part of the SPIRARE I clinical study.






