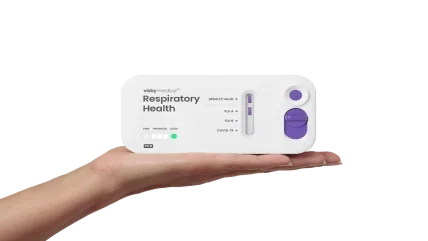
Visby Medical has secured 510(k) clearance, along with a CLIA waiver, from the US Food and Drug Administration (FDA) for its point-of-care respiratory health test.
The Visby Medical Respiratory Health Test is a rapid polymerase chain reaction (PCR) test that detects and differentiates between upper respiratory infections caused by influenza (Flu) A and B, and COVID-19.
This handheld, multiplexed molecular device is said to be the first of its kind to gain FDA clearance, following its emergency use authorisation (EUA) in December 2022.
The Visby Medical platform uses true-PCR technology, which is considered the gold standard for testing influenza A, influenza B, and COVID-19.
According to Visby Medical, the test delivers accurate results in under 30 minutes at the point of care. It enables clinicians to diagnose and treat patients in remote and resource-limited settings where laboratory services are less accessible.
In March 2023, the company received 510(k) clearance, as well as a CLIA waiver, from the FDA for its second-generation point-of-care (POC) test, the Visby Medical Sexual Health Test for women.
Visby Medical chief medical officer Gary Schoolnik said: “This FDA decision ensures that accurate, rapid testing with the Visby Medical Respiratory Health Test will remain available to help physicians quickly diagnose and treat patients as they face upcoming respiratory seasons.
“Fast diagnosis of patients with respiratory symptoms, enabling selection of the most appropriate treatments, is increasingly important to the medical community.”
This project is supported by federal funds from the US Department of Health and Human Services, the Administration for Strategic Preparedness and Response, and the Biomedical Advanced Research and Development Authority (BARDA).
Ongoing BARDA-funded studies aim to advance the development of at-home respiratory test features to enhance patient care and management.
Founded in 2012, Visby Medical is focused on infectious disease diagnosis and treatment, enabling clinicians to test, consult, and treat patients in a single visit.
Last month, the company secured an additional $3.9m from the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X).






